EVO ICL™ - EVOlution in Visual Freedom
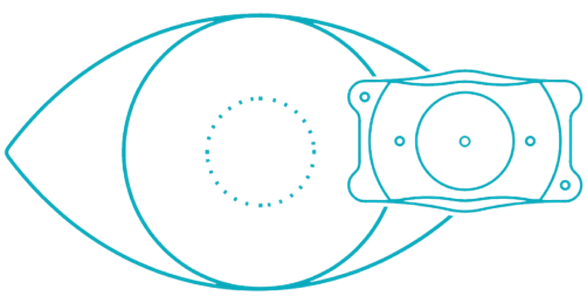
What is EVO ICL Lens?
EVO ICL is an Implantable Collamer Lens (ICL) designed to correct nearsightedness with and without astigmatism. The small flexible lens is placed behind the iris (the colored part of the eye) and in front of the natural crystalline lens in order to improve vision. It's like having your doctor implant a lens similar to a contact lens into your eye so you can live without the daily hassles and maintenance of typical contact lenses.

What to Expect During Your EVO ICL Procedure
EVO ICL lens is made of biocompatible Collamer material that works in harmony with your eye. Your eye doctor will create a small opening in your cornea that will be used to insert and position the EVO ICL. For most patients, the procedure is virtually painless with numbing eye drops and it typically takes less than 20-30 minutes. Patients typically experience improved vision right away and a quick recovery time.
Benefits of EVO ICL
Provides clear, sharp vision (1,2)
20 to 30-minute procedure
Quick recovery time
Removable by your doctor
Offers UV protection
Not visible once in place
Can treat nearsightedness with or without astigmatism
For patients who may not be candidates for LASIK or other vision correction procedures due to thin corneas (3,4)
Does not cause dry eye syndrome (5)
Excellent vision both day and night (6,7)
Over 2 million ICLs distributed worldwide
99.4% of patients surveyed would have the EVO Lens procedure again. (8)
Cost and Financing for EVO ICL
The cost of an EVO ICL procedure can vary depending on your personalized treatment plan. Many EVO ICL patients look forward to spending less in the long run with the EVO ICL compared to the continued costs of contacts and glasses. Ask about financing options and payment plans that may be available for your EVO ICL procedure.
Is EVO ICL Right for Me?
EVO ICL is well-suited for a wide variety of people. Ideal candidates for EVO ICL are aged 21 to 45 with nearsightedness, with or without astigmatism, and have maintained a stable prescription for at least one year. If you are seeking a solution to decrease or eliminate the cost and frustration of traditional contacts or glasses, EVO may be your answer. Contact us to discuss if EVO ICL is right for you.
Find a Grene Vision Group Eye Care Center Near You
Open your eyes to the advanced technology and professional service of Grene Vision Group. We have been serving the greater Wichita area communities with world-class eye care for nearly 100 years. We offer the most advanced technologies available, delivered by a committed, caring, and expert group of doctors and staff. Grene Vision Group is here for the lifetime of your family’s eye care needs.
Frequently Asked Questions About The EVO Visian ICL Lens
People who want a flexible and biocompatible vision correction option, as well as many patients who are not candidates for laser vision correction, find that Visian EVO ICL is the right procedure for them.
Ideal candidates for EVO Visian ICL:
Are between the ages of 21 and 45
Are seeking a vision correction solution for moderate to severe nearsightedness (with or without astigmatism)
Have a stable prescription that has not changed within the last year
Have good ocular health with no history of glaucoma, iritis, or diabetic eye disease
Vision correction with the EVO Visian ICL is an outpatient procedure that typically takes 20 to 30 minutes. After an initial exam with your ophthalmologist to determine that the EVO ICL is right for you, you will schedule your procedure. On the day of the procedure, your eyes will be numbed with an anesthetic eye drop and your refractive surgeon will implant the ICL between the iris (colored part of the eye) and the natural lens. Your surgeon will likely schedule a follow-up appointment the same day as the procedure.
Yes; the EVO Visian ICL is intended to provide permanent vision correction, but it can be removed or upgraded by your surgeon.
No, patients should not feel the lens as it does not adhere to any structures within the eye and does not move once in place.
No, the Visian EVO lens is placed behind your iris where only your eye doctor will be able to detect it.
Any medical procedure has certain risks associated with it. Potential complications of the EVO ICL procedure, although rare, include inflammation, an increase in eye pressure, or the need for an additional procedure, like an ICL exchange. While these potential risks are mitigated as much as possible, they can all typically be addressed early and without long-term side effects.
Important Safety Information
The EVO Visian ICL lens is intended to correct/reduce nearsightedness between -3.0 D up to -20.0 D and treat astigmatism from 1.0 D to 4.0 D. If you have nearsightedness within these ranges, EVO Visian ICL surgery may improve your distance vision without eyeglasses or contact lenses. Because the EVO Visian ICL corrects for distance vision, it does not eliminate the need for reading glasses, you may require them at some point, even if you have never worn them before. Since implantation of the EVO Visian ICL is a surgical procedure, before considering EVO Visian ICL surgery you should have a complete eye examination and talk with your eye care professional about EVO Visian ICL surgery, especially the potential benefits, risks, and complications. You should discuss the time needed for healing after surgery. Complications, although rare, may include need for additional surgical procedures, inflammation, loss of cells from the back surface of the cornea, increase in eye pressure, and cataracts. You should NOT have EVO Visian ICL surgery if your doctor determines that 1) the shape of your eye is not appropriate, 2) you do not meet the minimum endothelial cell density for your age at the time of implantation, 3) you have moderate to severe glaucoma, 4) your vision is not stable; or 5) if you are pregnant or nursing.
For additional information with potential benefits, risks and complications please visit DiscoverICL.com
1. Sanders D, Vukich J. Comparison of implantable collamer lens (ICL) and laser-assisted in situ keratomileusis (LASIK) for Low Myopia. Cornea. 2006
2. Igarashi A, Kamiya K, Shimizu K, Komatsu M. Visual Performance after implantable Collamer lens implantation and wavefront-guided laser in situ keratomileusis for high myopia. Am J Opthalmol. 2009
3. Parkhurst G, Psolka M, Kezirian G. Phakic intraocular lens implantation in United States military warfighters: A retrospective analysis of early clinical outcomes of the Visian ICL. J Refract Surg. 2011;27(7):473-481
4. Gimbel H, et al. Management of myopic astigmatism with phakic intraocular lens implantation. Journal of Cataract & Refractive Surgery, Volume 28, Issue 5, 883 – 886.
5. Ganesh S, Brar S, Pawar A. Matched population comparison of visual outcomes and patient satisfaction between 3 modalities for the correction of low to moderate myopic astigmatism. Clin Ophthalmol. 2017 Jul 3;11:1253-1263.
6. Parkhurst GD. A prospective comparison of phakic collamer lenses and wavefront-optimized laser-assisted in situ keratomileusis for correction of myopia. Clin Ophthalmol. 2016 Jun 29;10:1209-15.
7. Ganesh S, Brar S, Pawar A. Matched population comparison of visual outcomes and patient satisfaction between 3 modalities for the correction of low to moderate myopic astigmatism. Clin Ophthalmol. 2017 Jul 3;11:1253-1263.
8. Packer M. The Implantable Collamer Lens with a central port: review of the literature. Clinical Ophthalmology 2018: 12: 2427–2438

 316-636-2010
316-636-2010 316-609-2177
316-609-2177